Fiscal 2006
(1) Hyper-Raman Microspectroscopy: A New Approach for Completing Vibrational Information under a Microscope
Raman microspectroscopy, both linear and non-linear, is now extensively used as a unique and powerful method for studying heterogeneous molecular systems in situ or in vivo. Sub-micrometer spatial resolution is readily achievable with a con-focal microscopic configuration. On the other hand, infrared microspectroscopy does not compare with Raman, because of its low spatial resolution (5—10 μm) due to the diffraction limit. We have developed a new vibrational microspectroscopic method equivalent to infrared, using hyper-Raman (HR) scattering. HR scattering has its own selection rules that are different either from infrared absorption or Raman scattering. Infrared active vibrations are all HR active and Infrared/Raman inactive vibration can be HR active. Figure 1 shows a HR image of a β-carotene micro-crystal obtained with the infrared active but Raman-inactive C=C stretch band at 1564 cm-1. The exciting laser wavelength is 800 nm and the estimated lateral and depth resolutions are 0.6 μm and 1.4 μm. We are now able to observe infrared-active vibrations under a microscope by using HR scattering, while keeping the high spatial resolution characteristic of visible laser microspectroscopy.
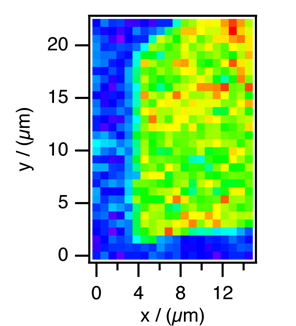
Fig. 1 Hyper-Raman image of a microcrystal of β-carotene.
(2) Structure and Electric Dipole Moments of Solvated p-Nitroanilines in CH3CN/CCl4 as Studied by Infrared Electroabsorption Spectroscopy
We have previously shown that p-nitroaniline (pNA) in CH3CN/CCl4 forms two distinct salvation structures, 1:1 and 1:2 forms, with one and two CH3CN attached to pNA. Infrared electroabsorption spectroscopy detects absorption changes due to the orientational and electronic polarizations of molecules under an applied electric field. The former gives quantitative information on the dipole moment, while the latter on the molecular polarizability. These two signals can be separated by measuring the χdependence of the signal, where χ is the angle between the external electric field and the incident infrared light field. The observed χdependence of pNA in CH3CN/CCl4 is shown in Fig. 2. From the singular value decomposition analysis of this result combined with an ab initio MO calculation (HF/6-31+G** level), the structure and dipole moments of the two forms has been determined as shown in Fig. 3.
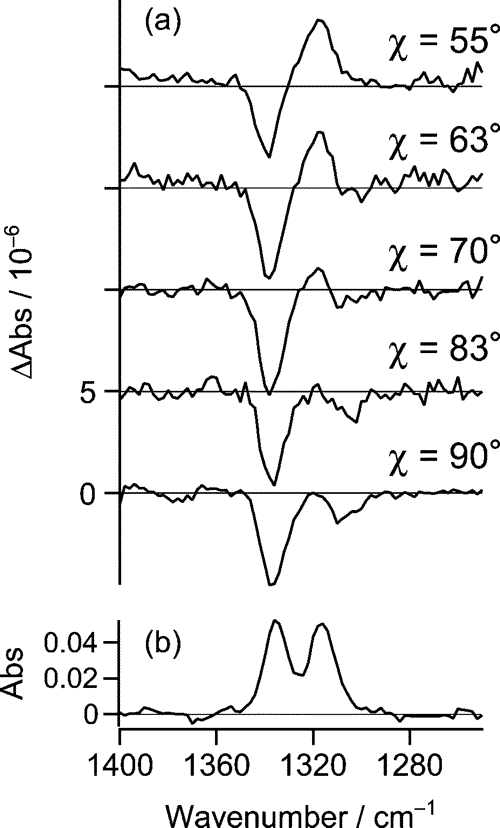
Fig. 2 The observed χdependence of infrared electroabsorption of pNA in CH3CN/CCl4 mixed solvent.
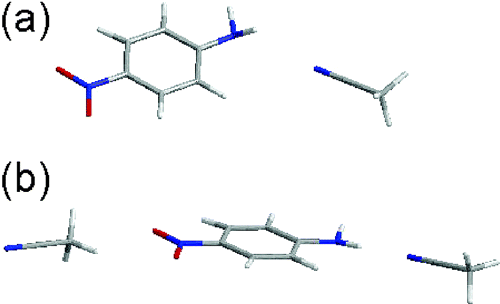
Fig. 3 The structure of the 1:1 form (a) and that of 1:2 form (b).
Refereed Journals
- Raman spectra indicative of unusual water structure in crystals formed from a room-temperature ionic liquid. Hiroko Miki, Satoshi Hayashi, Hiroshige Kikura and Hiro-o Hamaguchi, J. Raman Spectrosc., 37, 1242-1243 (2006).
- Vibrational imaging of a J-aggregate microcrystal using ultrabroadband multiplex coherent anti-Stokes Raman scattering microspectroscopy. Hideaki Kano and Hiro-o Hamaguchi, Vibrational Spectrosc., 42 (1) 135-139 (2006).
- Magnetic manipulation of materials in a magnetic ionic liquid. Masanari Okuno, Satoshi Hayashi and Hiro-o Hamaguchi, Appl. Phys. Lett., 89, 132506 (2006).
- Evidence for mesoscopic local structures in ionic liquids: CARS signel spatial distribution of C(n)mim[PF6] (n=4,6,8). Shinsuke Shigeto and Hiro-o Hamaguchi, Chem. Phys. Lett., 427, 329-332 (2006).
- Ion association dynamics in aqueous solutions of sulfate salts as studied by Raman band shape analysis. DaisukeWatanabe and Hiro-o Hamaguchi, J. Chem. Phys., 124, 247102 (2006).
- Heat capacity and glass transition of an ionic liquid 1-butyl-3-methylimidazolium chloride. Osamu Yamamuro, Y.Minamimoto, Y. Inamura, Satoshi Hayashi and Hiro-o Hamaguchi, Chem. Phys. Lett., 423, 371-375 (2006).
- Vibrationally resonant imaging of a single living cell by supercontinuum-based multiplex coherent anti-Stokes Raman Scattering microspectroscopy. Hideaki Kano and Hiro-o Hamaguchi, Optics Express 13, 1322-1327 (2005).
- In-vivo multi-nonlinear optical imaging of a living cell using a supercontinuum light source generated from a photonic crystal fiber. Hideaki Kano and Hiro-o Hamaguchi, Optics Express 14, 2798-2804 (2006).
- Structure and dipole moments of the two distinct solvated forms of p-nitroaniline in acetonitrile/CCl4 as studied by infrared electroabsorption spectroscopy. Shinsuke Shigeto, Hirotsugu Hiramatsu and Hiro-o Hamaguchi, J. Physical Chem. A, 110 (13) 3738-3743 (2006).
- Vibrational Imaging of a Single Pollen Grain by Ultrabroadband Multiplex Coherent Anti-Stokes Raman Scattering Microspectroscopy. Hideaki Kano and Hiro-o Hamaguchi, Chem. Lett., 35 (10) 1124-1125 (2006).
- Molecular near-field effect and intensity enhancement of solvent modes in resonance hyper-Raman sattering. Rintaro Shimada, Hideaki Kano and Hiro-o Hamaguchi, J. Raman Spectrosc., 37, 469-471 (2006).
- Effect of water on the molecular structure and arrangement of nitrile-functionalized ionic liquids. Satyen Saha and Hiro-o Hamaguchi, J. Phys. Chem. B, 110 (6) 2777-2781 (2006).
- Dispersion-compensated supercontinuum generation for ultrabroadband multiplex coherent anti-Stokes Raman scattering spectroscopy. Hideaki Kano and Hiro-o Hamaguchi, J. Raman Spectrosc., 37, 411-415 (2006).
- Three-Dimensional Vibrational Imaging of a Microcrystalline J-Aggregate Using Supercontinuum-Based Ultra-Broadband Multiplex Coherent Anti-Stokes Raman Scattering Microscopy. Hideaki Kano and Hiro-o Hamaguchi, J. Phys. Chem. B, 110 3120-3126 (2006).
- Hyper-Raman microspectroscopy: a new approach to completing vibrational spectral and imaging information under a microscope. Rintaro Shimada, Hideaki Kano and Hiro-o Hamaguchi, Optics Lett., 31 (3) 320-322 (2006).
- A new nonlinear Raman probe for local structures in liquids and solutions. Shinsuke Shigeto and Hiro-o Hamaguchi, Chem. Phys. Lett., 417, 149-153 (2006).
- A new class of magnetic fluids: bmim[FeCl4] and nbmim[FeCl4] ionic liquids. Satoshi Hayashi, Satyen Saha and Hiro-o Hamaguchi, IEEE Transactions on Magnetics, 42, 12-14 (2006).