Fiscal 2002
(1)"Rotation around a Double Bond Takes Place in the Excited Electronic State; The Dynamic Polarization Model of Photoisomerization"
Rotation around a C=C double bond is prohibited in the ground electronic state at room temperature, giving rise to the trans/cis geometrical isomerism. If we photoexcite the double bond electronically, however, the rotation becomes feasible and conversion between the trans and cis isomers takes place (photoisomerization). We previously showed that the central carbon-carbon bond of trans-stilbene in the S1 state, where the isomerization actually proceeds, is a double bond and not a single bond. The rotation certainly occurs around the double bond of S1 trans-stilbene. Why and how? From the analysis of a newly found linear relatioship between the solvent dependent C=C stretch frequency and the rate of isomerization, we proposed a model in which occasional polarization of the C=C double bond into a C+-C- (or C-C) single bond facilitates the rotation (Fig. 1). The polarization is induced dynamically by solvent fluctuation (solvent induced dynamic polarization). Recently, we obtained further experimental support for this model. Fig. 2 shows the plot of the C=C stretch frequency of S1 trans-stilbene vs the rate of isomerization kiso in three different solvents (hexane, octane and decane) at five different temperatures. The plot makes five straight lines converging to the same point at the zero isomerization rate. This result is fully consistent with our model of dynamic polarization.
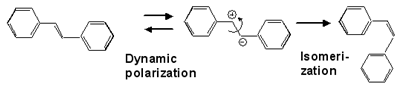
Fig. 1
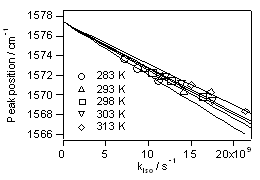
Fig. 2
(2)"Ultrafast Stepwise Double Proton Transfer Reaction in the Excited State of 2-Aminopyridine/Acetic Acid Complex"
Proton relay, in which a proton is transported by successive transfers between prearranged donor/accepter pairs, is involved in many biological processes. It plays an important role in producing a pH gradient across a membrane that facilitates the ATP synthesis. Photoinduced double proton transfer has been attracting much attention as a prototype model of the proton relay. There has been a controversy over the last decade as to whether the double proton transfer proceeds in a concerted way (two protons move simultaneously) or in a stepwise fashion (two protons move one after another). We studied the photoinduced double-proton-transfer tautomerization reaction in the excited state of the 2-aminopyridine/acetic acid complex by picosecond time-resolved fluorescence spectroscopy. It has been found that, immediately after the photoexcitation, the proton of the acetic acid moiety transfers to the ring nitrogen of 2-aminopyridine to form the protonated cation radical of 2-aminopyridine and then the proton of the amino group transfers, with a time constant of 5 ps, to the acetic acid moiety to complete tautomerization (Fig. 3). This is the first example of a stepwise double proton transfer reaction in which the zwitterionic intermediate is identified unambiguously by time-resolved spectroscopy. The rate of the second proton transfer shows a marked temperature effect but the behavior is somewhat extraordinary; there is no appreciable H/D isotope effect on the activation energy obtained from the Arrhenius plot. No existing theory can account for this puzzling result. We are now trying to solve the problem by a new theoretical approach that considers the solvent induced dynamic polarization of the zwitterionic intermediate.
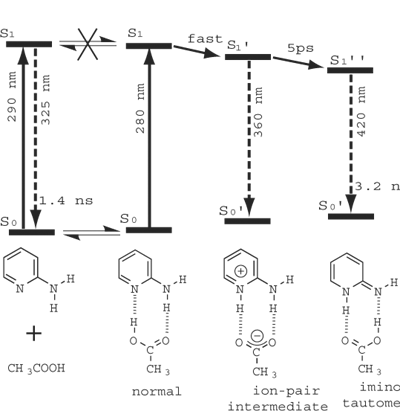
Fig. 3