Fiscal 1998
(1) "Ultra fast physical-chemical process of retinal"
The photoisomerization of retinal shows marked solvent dependence of the quantum yield and the product isomer distribution. It raises an interesting question of how the solvent/solute interaction intervenes in the excited intermediate state and thereby affects the branching of the reaction. We used femtosecond time-resolved visible and ultraviolet absorption spectroscopy and studied the photoisomerization of all-trans and 9-cis retinal in a non-polar solvent, hexane. For all-trans retinal, three singlet excited states, S3, S2, S1, were observed in the time-resolved visible absorption spectra and their dynamics determined. An isomerization pathway through the S2 state has been deduced from the ground-state recovery dynamics in the time-resolved ultraviolet absorption spectra. Three singlet excited states were also observed for 9-cis retinal. These results, combined with the known photoisomerization pathway on the triplet potential surface, led to a global picture of the photophysics and photochemistry of retinal as shown in Figure 1. Recent time-resolved visible absorption study in butanol indicated the formation of a strong hydrogen-bonding in the S2 all-trans retinal, which accounts very well for the strong solvent effect of alcohols on the quantum yield and the product distribution of the retinal photoisomerization.
J. Chem. Phys., 109, 1397-1408 (1998)
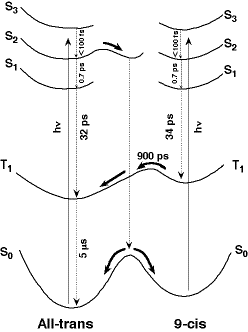
Fig. 1. Photophysics and photochemistry of retinal
(2) "A microscopic view of the solvent effect"
Many interesting molecular phenomena in solution, including the solvatochromism of para-nitroaniline (pNA), are categolized as "the solvent effect". However, the meaning of "the solvent effect" is scant, or rather, its elucidation is one of the central problems of modern physical chemistry. The solvatochromism of pNA is explained in terms of the solvent polarity; the charge-transfer character of the pNA molecule changes with the solvent polarity and so does the electronic absorption. We studied the solvatochromizm of pNA in acetonitrile(AN)/CCl4 mixed solvents using ultraviolet absorption spectroscopy. As expected, we observed a marked bathochromic shift of the electronic absorption with increasing AN concentration. However, an SVD analysis of the spectral change indicated that it should be ascribed to the three distinct solvated structures of pNA, the 1:2, 1:1, and 1:0 forms (Figure 2), which exist in varying abundances. These three solvated forms have distinct absorption spectra reflecting step-wise changes of the charge-transfer character. Thus, the macroscopic (dielectric) polarity does not explain the solvatochromism of pNA in AN/CCl4 . In neat AN, only the 1:2 and 1:1 forms are present. By using a 300-fs laser pulse at 400 nm, we were able to selectively photoexcite the 1:2 species and observed the subsequent dynamics. The results showed that the 1:2 form dissociates to the 1:0 form much faster than the experimental time resolution and that the recombination takes place directly to the 1:2 form with a time constant of 700 fs. The pNA/AN/CCl4 system provides a useful model for studying "the solvent effect" in terms of the structure and dynamics of the solute/solvent complex or supramolecule.
Laser Chem., 19, 329-333 (1999)
Bull. Chem. Soc. Jpn., A 72, 389-395 (1999)
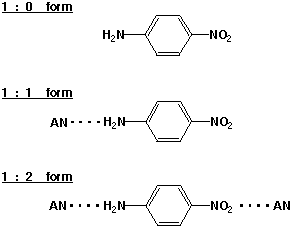
Fig. 2. Three distinct solvated structures of pNA in AN/CCl4