Fiscal 2003
(1) "Molecular-level Pursuit of Yeast Mitosis by Time- and Space-resolved Raman Spectroscopy"
Yeast is the simplest eukaryote that serves as a prototype model for studying a wide variety of biological phenomena at the cellular level. Fission yeast (Schizosaccharomyces pombe) is a kind of yeast, which repeats cell division according to the cycle G2→M→G1→S→G2. Although a number of detailed microscopic and electron-microscopic observations have been reported, molecular-level tracing of yeast mitosis in vivo was not possible till the present time. We have succeeded in recording the time- and space-resolved Raman spectra (time resolution: 100 s, spatial resolution: 250 nm) of a dividing S.pombe cell on a confocal Raman microspectrometer. We used an S.pombe cell whose nuclei were labeled by GFP so that the position and the number of nuclei were directly seen as fluorescence images. Figure 1 shows the Raman spectra of the central part (the area surrounded by small red circles) of an S.pombe cell in the M to G2 period of the mitosis. The Raman spectrum of the dividing nucleus at the M period (0 min) is dominated by known protein bands. As the mitosis proceeds, the spectrum changes to that of mitochondria, which contain many strong bands of phospholipids, then it further changes to that of septum and finally to that of the cell wall. This is the first in vivo observation of the dynamic behavior of biomolecules during the cell division.
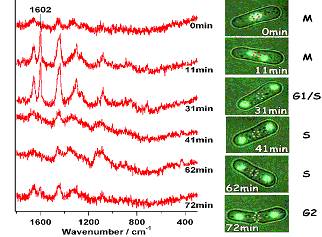
Fig. 1. Time- and space-resolved Raman spectra of a dividing fission yeast cell.
(2) "Novel Nano Structure in Ionic liquids: Are Ionic Liquids Genuine Liquids?"
Liquids that are composed solely of ions are called ionic liquids. They attract much attention of chemists for their potential applications as new "green" solvents and/or as new electrochemical materials having wide potential windows. Nevertheless, the physicochemical nature of ionic liquids is not well understood. The reason why they are liquids is yet to be elucidated. We have studied systematically the liquid and crystal structures of protoype ionic-liquid compounds, 1-butyl-3-methyl-imidazolium halides bmim X, where X=Cl, Br, I. We found the crystal polymorphism of bmim Cl and identified the two crystal plolymorphs, Crystal (1) and Crystal (2), by powder x-ray diffraction and Raman spectroscopy. We prepared single crystals of bmim Cl Crystal (1) and bmim Br, and determined their crystal structures (Fig. 2). Both crystals were shown to have chracteristic molecular arrangements in which the imidazolium cations make columns extending along the crystal a axis and two chains of the halgen anions are accommodated in a space surrounded by four cation columns. Raman spectra and their temperature dependence suggested that those characteristic column structures may also exsit, at least partially, in the ionic liquid state. Wide-angle x-ray scattering of liquid bmim I confirmed periodical arrangements of the iodide anions. All these results indicate that ionic liquids are entirely new class of fluids that are beyond the conventional concept of molecular liquids.
1-7) Chem. Lett. 32, 740-741 (2003).
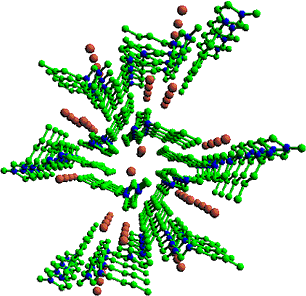
Fig. 2. Molecular arrangements in bmin Cl Crystal (1) as determined by single-crystal X-ray analysis.