Fiscal 2001
(1)"Development of Infrared Electroabsorption Spectroscopy and Its Application to Structural Studies in Liquids and Solutions"
Electrostatic interactions play key roles in many of the molecular phenomena occurring in liquids and solutions. Spectroscopic probing of the molecular responses to an applied external electric field provides useful information on those electrostatic interactions. We have developed infrared electroabsorption spectroscopy in which molecular responses to the external electric field are probed by infrared absorption. Infrared electroabsorption spectrum in the 1400 cm-1 - 850 cm-1 region of liquid 1,2-dichloroethane is shown in Figure 1, together with the ordinary infrared absorption spectrum. The observed electroabsorption signals (a) are found to consist of two components, the zeroth-derivative component (b) and the first-derivative component (c). The former arises from the reorientational polarization of the gauche conformer and the electric-field-induced shift of the trans-gauche equilibrium. The latter comes from the electronic polarization. From the analysis of the zeroth-derivative signal, the free-energy difference DG between the trans and the gauche conformers has been determined experimentally for the first time. The DG value has then been converted to the entropy difference DS with the use of the known enthalpy difference DH. The obtained DS value indicates that, reflecting the dipole-dipole interactions, the translational motion of the gauche conformer is more restricted than that of the trans in liquid 1,2-dichloroethane.
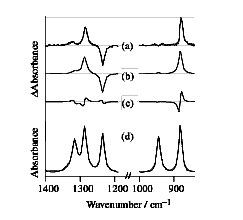
Fig. 1
(2)"Photoisomerization Proceeds in an Ionic Liquid?"
Liquids that are composed solely of ions are called ionic liquids. Ionic liquids provide an interesting model system in which the nature of liquids may manifest itself. They attract much attention as a new class of solvents that can produce extraordinary molecular environments, as well as new functional liquids for electrochemical applications. We are interested in the fundamental physicochemical properties of ionic liquids, including their structure, dynamics, and electrical and mechanical properties as solvents. Photoisomerization of trans-stilbene is one of the most extensively studied elementary photochemical processes and is known to be affected profoundly by the solvent. In a viscous solvent, the rotation around the central C=C linkage is thought to be hindered to reduce the reaction rate. It is therefore of considerable interest to see whether the photoisomerization of trans-stilbene proceeds in an ionic liquid, 1-butyl-3-methylimidazolium hexafluorophosphate (bmimPF6), whose viscosity is 312 cP at 303 K and is more than two orders of magnitude larger than those of typical organic solvents. We found that the photoisomerization of trans-stilbene indeed proceeds in bmimPF6 with a rate of kiso=6.6×109 s-1. If we extrapolate the viscosity dependence of the isomerization rate in normal alcohols, which have comparable polarity as bmimPF6, the value kiso=9.7×108 s-1 is obtained (Fig. 2). The observed rate is about an order of magnitude larger than the extrapolated value, indicating that the conventional framework based on polarity and viscosity is not applicable to ionic liquids and that a new molecular-level approach is necessary to fully understand ionic liquids as solvents.
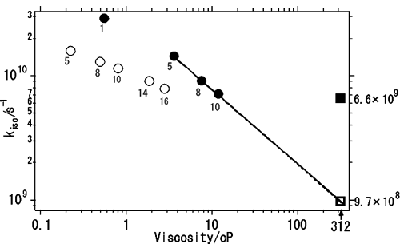
Fig. 2